PGx Pharmacogenetic Testing
What is PGx
All drugs can cause adverse side-effects to a greater or lesser degree but imagine if you had a test that could identify the likelihood of severe reactions or guide you to prescribe the most suitable drug & effective dose!
Pharmacogenetics, often referred to as PGx, is a new diagnostic tool that identifies the presence of genetic variants in patients, that impact drug response, efficacy and safety. PGx can become a powerful tool to help clinicians avoid the trial-and-error process and prescribe medications more safely and effectively, especially in the area of psychiatry. For example, many patients achieve remission with their first antidepressant, while up to 40% of them endure weeks or months of ineffective treatment before trying another medication. Psychiatric medications include anti-depressants, anti-anxiety and anti-psychotic medications.



When to Use a PGx Test
While the benefits in Psychiatry are particularly significant, our PGx test supports prescribing in Cardiology, Oncology and many other indications where gene–drug interactions are increasingly recognised as central to personalised therapy.
Our PGx report is based on international guidelines (e.g. CPIC, DPWG, SEFF) and recommendations from regulatory authorities (e.g. EMA, FDA, Swissmedic). Exclusively highly actionable and evidence based information of CPIC levels A & B, ClinPGx levels 1 & 2 are being used.
Learning About PGx
The British Pharmacological Society (BPS) in collaboration with CERSI-PGx have developed an educational Portal focused on upskilling stakeholders on Pharmacogenomic testing.
Find out more about CERSI-PGx: https://cersi-pgx.org/
Visit the free eLearning portal: https://lnkd.in/exSGD_dH
Actionable for GPs
- Clear, concise report designed for immediate clinical use in primary care
- Includes two report formats:
- A patient-friendly version in plain language
- A clinician version with evidence-based recommendations and clinical implications
- Direct access to a clinician support webpage with alternative medication guidance where PGx risks are identified.
Clinically Robust & Future-Proof
- Evidence base is reviewed and updated quarterly to reflect the latest science
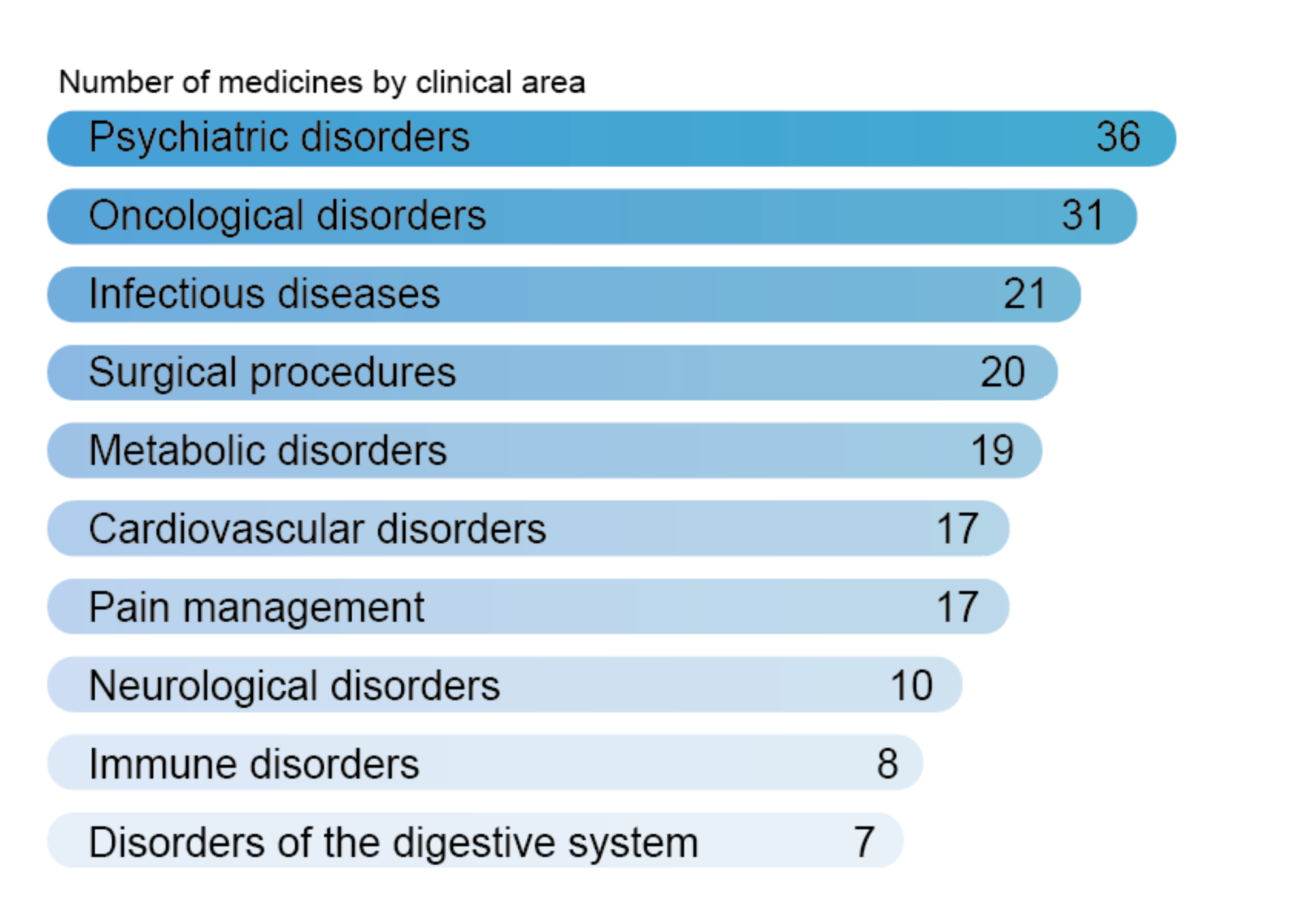
- We test 27 key genes impacting 119 commonly prescribed medicines
- Our test is a Pre-emptive test
- Results remain relevant for future prescriptions, not just current therapy

Benefits
30% Reduction of Adverse Drug Reactions (ADRs) through PGx
In addition drug-drug interactions as well as weight, age, lifestyle and other external environmental factors will also impact the reaction to medicines.
How PGx helps you
Prescribing the right medication on the first try is challenging. Our PGx report shows which drugs are likely to cause side effects or lack of efficacy based on each patient’s genetic profile.
Sorting of medicines by severity level helps you to avoid high-risk options and tailor dosing.
Side Effect Category
- Severe Effect
- Moderate Effect
- Mild Effect
- Medicines not affected

PGx Videos
Typically, DNA variants affect the enzymes that metabolise (CYP family) and transport drugs or affect the defense system. Our panel based PGx test uses SNP genotyping arrays to identify single nucleotide variants (SNVs) and copy number variations (CNVs) in 27 genes or gene combinations in your patient.

What is involved in PGx?
The patient will be provided with a saliva collection tube or a blood collection tube and postage instructions.

TAT
Test results are provided in 2 to 3 weeks.
Precautions
This test is not suited to patients who have had -
- A Blood Transfusion in the previous 4 weeks
- A Bone Marrow, Stem Cell, Liver or Kidney Transplant

References
1. Kraus, C. et al. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry 9, 127 (2019). https://doi.org/10.1038/s41398-019-0460-3
2. Jeffrey R. Strawn et al. Adverse Effects of Antidepressant Medications and their Management in Children and Adolescents. Pharmacotherapy, 43, 7, pages 675-690.https://doi.org/10.1002/phar.2767
3. Fabbri, C., Serretti, A. Pharmacogenetics of Major Depressive Disorder: Top Genes and Pathways Toward Clinical Applications. Curr Psychiatry Rep 17, 50 (2015).
4. Swen J. et al. 2023. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. The Lancet.
5. Ji, Y. et al. (2016) Preemptive Pharmacogenomic Testing for Precision Medicine : A Comprehensive Analysis of Five Actionable Pharmacogenomic Genes Using Next-Generation DNA Sequencing and a Customized CYP2D6 Genotyping Cascade. The Journal of molecular Diagnostics. 18(3), 438-445., 95(4), 423-432. https://doi.org/10.1016/j.jmoldx.2016.01.003
6. Bertollo, A.G.; Mocelin, R.; Ignácio, Z.M. Pharmacogenetics and the Response to Antidepressants in Major Depressive Disorder. Pharmaceuticals 2025, 18, 1360. https://doi.org/10.3390/ph18091360
7. NHS Digital. Prescription cost analysis data: NHS Digital; 2019. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018. Accessed May 29, 2020.
8. Taylor S, Annand F, Burkinshaw P, et al. Dependence and Withdrawal Associated with Some Prescribed Medicines: An Evidence Review. London: Public Health England; 2019.
9. McCool A et al. Antidepressant medication prescribing patterns in Irish general practice from 2016 to 2020 to assess for long-term use. Ir J Med Sci. 2022 Oct;191(5):2239-2246. doi: 10.1007/s11845-021-02833-7. Epub 2021 Oct 29. PMID: 34714490; PMCID: PMC8554180.
10. Ghanbarian S, et al. Cost-effectiveness of pharmacogenomic-guided treatment for major depression. CMAJ. 2023 Nov 14;195(44):E1499-E1508. doi: 10.1503/cmaj.221785. PMID: 37963621; PMCID: PMC10699479.